|
 |
- Search
| Neonatal Med > Volume 29(4); 2022 > Article |
|
Abstract
Purpose
Erythropoietin (EPO) is a promising neuroprotective drug. We investigated whether EPO has beneficial effects on neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy (HIE).
Methods
We retrospectively reviewed the data of 56 infants with HIE born at or after 35 weeks of gestation who were admitted to Inha University Hospital between 2012 and 2021. Patients were divided into two groups based on EPO use and compared. In the EPO group, patients were administered 1,000 U/kg of EPO on days 1, 2, 3, 5, and 7, starting within 24 hours after birth. The primary outcome was death or neurodevelopmental impairment (NDI) at the age of 12 months.
Results
EPO was administered to 38 infants, and 18 did not receive EPO. Only 37.5% of patients with HIE (21/56) and 60% of patients with moderate-to-severe HIE (21/35) received therapeutic hypothermia. Among all patients with HIE, death or NDI (21.1 % vs. 50.0%; odds ratio [OR], 0.09; 95% confidence interval [CI], 0.01 to 0.78; P=0.029) and brain injury on imaging (42.1% vs. 83.3%; OR, 0.16; 95% CI, 0.03 to 0.92; P=0.040) were significantly lower in the EPO group than in the control group. Among patients with moderate-to-severe HIE, brain injury on imaging (54.2% vs. 90.9%; OR, 0.04; 95% CI, 0.002 to 0.700; P=0.027) was significantly lower in the EPO group than in the control group.
Hypoxic-ischemic encephalopathy (HIE) is a major cause of neonatal death and neurodevelopmental impairment (NDI) [1,2]. Because perinatal asphyxia is unpredictable and difficult to prevent, adequate and timely treatment of affected newborns is essential for neuroprotective management of neonatal HIE [2,3]. Therapeutic hypothermia is the only proven treatment for neonatal HIE [2-4]. However, not all neonates with HIE are eligible for therapeutic hypothermia, and there are still cases of treatment failure.
Recently, erythropoietin (EPO) has been highlighted as a promising neuroprotective agent in neonatal HIE. In a hypoxic state, EPO production increases in brain cells, which plays an important role in neuroprotection and neuroregeneration in the central nervous system [5,6]. Furthermore, increasing evidence suggests that EPO administration reduces mortality and improves neurodevelopmental outcomes in infants with HIE [7-13].
The time has come to discuss the use of EPO for neonatal HIE in the real clinical situation in Korea. Therefore, we investigated whether EPO administration has a beneficial effect on mortality and neurodevelopmental outcomes in newborns with HIE.
We retrospectively reviewed the data of infants with HIE born at a gestational age of ≥35 weeks who were admitted to the neonatal intensive care unit (NICU) of Inha University Hospital from 2012 to 2021. The inclusion criterion for HIE was altered levels of consciousness with perinatal asphyxia. Altered consciousness was defined as having at least one of the following: lethargy, stupor, coma, hyperalert state, hypotonia, abnormal reflexes including oculomotor or pupillary abnormalities, absent or weak suck, or clinical seizures [14]. Perinatal asphyxia was based on at least one of the following criteria: 10 minutes Apgar score of ≤5; the need for resuscitation at 10 minutes (i.e., chest compressions or endotracheal or mask ventilation); pH <7.00 or base deficit ≥12 mEq/L in capillary or venous blood within 60 minutes of birth [15]. The 56 infants included in the study were divided into two groups according to EPO use. Off-label EPO was administered as per the attending clinician’s discretion with the parents’ consent. In the EPO group (n=38), EPO was administered intravenously at a dose of 1,000 U/kg on days 1, 2, 3, 5, and 7 of life, starting within 24 hours after birth [12]. This study was approved by the Institutional Review Board (IRB) of Inha University Hospital (IRB no. 2022-05- 017). The requirement for informed consent was waived by the board owing to the retrospective nature of the study.
Baseline characteristic data, including sex, gestational age, birth weight, delivery type, birthplace, Apgar scores, initial results of capillary or venous gas analysis, serum lactic acid, and lactate dehydrogenase, were collected. HIE was classified as mild, moderate, or severe based on the modified Sarnat and Sarnat staging [14]. In addition, patients' clinical courses during NICU admission, including seizures and therapeutic hypothermia, were reviewed. The primary outcome was death or NDI at 12 months. The secondary outcome was brain injury observed on imaging. NDI was defined as cerebral palsy, cognitive impairment, or severe visual or hearing impairment that required assistance at 12 months of age. Brain injuries were defined as any injury to at least one region of the brain, including the cortex, basal ganglia, thalamus, posterior limb of the internal capsule, white matter, brain stem, or cerebellum, identified on brain ultrasonography or brain magnetic resonance imaging (MRI). Brain ultrasonography was performed using an 8 to 12 MHz transducer during the 1st week of life by a radiologist blinded to the interventions. Coronal and sagittal sections were obtained using anterior fontanelles. Brain MRI with diffusion-weighted imaging was performed using SIGNATM (Architect-70, GE Healthcare, Chicago, IL, USA). All brain images were reviewed by two experienced radiologists who were blinded to the use of EPO. When more than one imaging study or report was available, the most severe report was selected.
Variables are expressed as median (interquartile range [IQR]) or number (%). We compared both groups using the Mann-Whitney U-test for continuous variables and Fisher’s exact test for categorical variables, as appropriate. Statistical analysis was performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P<0.05.
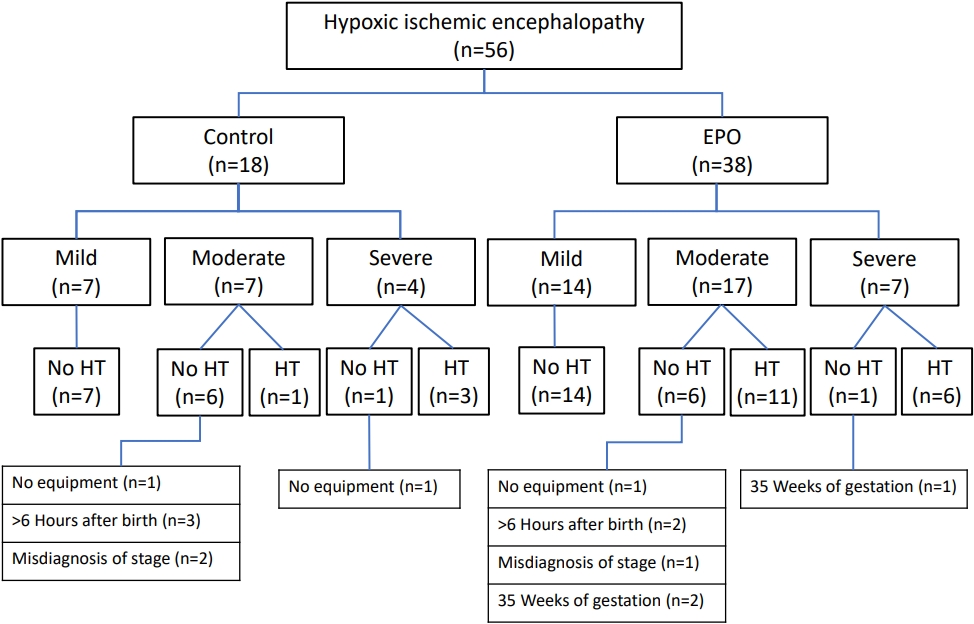
Of the 56 patients with HIE, 38 received EPO, and 18 did not (Figure 1). A total of 35 (62.5%) infants, including 21 (60%) infants with mild HIE and 14 (40%) with moderate-to-severe HIE, did not receive therapeutic hypothermia. The reasons for not receiving hypothermia among patients with moderate-to-severe HIE were as follows: no equipment available (n=3), >6 hours after birth (n=5), misdiagnosis as the mild stage of HIE on the 1st day (n=3), and <36 weeks of gestation (n=3). Brain ultrasonography was performed for all included patients. Brain MRI was performed in 46 patients (82%) on the median of 5 days (IQR, 4 to 7) of life. In the group comparison, although there were no differences in gestational age, birth weight, sex, maternal age, and mode of delivery, the Apgar score at 1 minute was lower (median 3 [IQR, 1 to 5] vs. 5 [IQR, 3 to 6], P=0.031), and the pCO2 at the initial capillary blood gas analysis was higher (69.7 cmH2O [IQR, 64.8 to 86.5] vs. 58.8 cmH2O [IQR, 33.5 to 68.5], P=0.009) in the EPO group than in the control group (Table 1). The frequency of clinical seizures did not differ between groups (52.6% vs. 44.4%, P=0.775), nor did as the mortality rate (15.8% vs. 22.2%, P=0.711). Death during neonatal hospitalization occurred in three patients in the EPO group, and three patients died at 3, 7, and 12 months of age after NICU discharge. In the control group, three patients died during NICU admission, and one patient died during follow-up at the age of 11 months. Death or NDI at 12-month-of-age was significantly lower in the EPO group (21.1% vs. 50%, P=0.041; odds ratio [OR], 0.09; 95% confidence interval [CI], 0.01 to 0.78; P=0.029) than in the control group (Tables 1, 2). Brain injury on imaging was also significantly lower in the EPO group (42.1% vs. 83.3%, P=0.004; OR, 0.16; 95% CI, 0.03 to 0.92; P=0.040) than in the control group.
Of the 35 included patients with moderate-to-severe HIE, 24 and <36 weeks of gestation (n=3). Brain ultrasonography was performed for all included patients. Brain MRI was performed in 46 patients (82%) on the median of 5 days (IQR, 4 to 7) of life. In the group comparison, although there were no differences in gestational age, birth weight, sex, maternal age, and mode of delivery, the Apgar score at 1 minute was lower (median 3 [IQR, 1 to 5] vs. 5 [IQR, 3 to 6], P=0.031), and the pCO2 at the initial capillary blood gas analysis was higher (69.7 cmH2O [IQR, 64.8 to 86.5] vs. 58.8 cmH2O [IQR, 33.5 to 68.5], P=0.009) in the EPO group than in the control group (Table 1). The frequency of clinical seizures did not differ between groups (52.6% vs. 44.4%, P=0.775), nor did as the mortality rate (15.8% vs. 22.2%, P=0.711). Death during neonatal hospitalization occurred in three patients in the EPO group, and three patients died at 3, 7, and 12 months of age after NICU discharge. In the control group, three patients died during NICU admission, and one patient died during follow-up at the age of 11 months. Death or NDI at 12-month-of-age was significantly lower in the EPO group (21.1% vs. 50%, P=0.041; odds ratio [OR], 0.09; 95% confidence interval [CI], 0.01 to 0.78; P=0.029) than in the control group (Tables 1, 2). Brain injury on imaging was also significantly lower in the EPO group (42.1% vs. 83.3%, P=0.004; OR, 0.16; 95% CI, 0.03 to 0.92; P=0.040) than in the control group. 2. Patients with moderate-to-severe HIE Of the 35 included patients with moderate-to-severe HIE, 24 received EPO, and 11 did not. There were no differences in gestational age, birth weight, sex, maternal age, or mode of delivery. The Apgar score at 1 minute was lower (median 2 [IQR, 1 to 4] vs. 5 [IQR, 3 to 5], P=0.049), whereas the pCO2 at the initial capillary blood gas analysis was higher (80.6 cmH2O [IQR, 67.3 to 97.3] vs. 51.3 cmH2O [IQR, 23.5 to 76.3], P=0.025) in the EPO group than in the control group (Table 1). Serum lactic acid levels were higher and lactate dehydrogenase levels were lower in the EPO group (12.6 mmol/L [IQR, 6.2 to 13.3] vs. 4.7 mmol/L [IQR, 2.1 to 6.9], P=0.025; and 632 U/L [IQR, 465 to 943] vs. 1,066 U/L [IQR, 912 to 1,244], P=0.047; respectively) than in the control group (Table 1). The proportion of infants who received therapeutic hypothermia was not different between groups (70.8% vs. 36.4%, P=0.073). The frequency of clinical seizures did not differ (79.2% vs. 72.7%, P=0.685), nor did the mortality rate (25.0% vs. 36.4%, P=0.689). Death or NDI at 12-month-of-age was lower in the EPO group than in the control group; however, the difference was not statistically significant (37.5% vs. 72.7%, P=0.050; OR, 4.6; 95% CI, 0.30 to 70.10; P=0.272) (Tables 1, 2). Brain injury on imaging was significantly lower in the EPO group (54.2% vs. 90.9%, P=0.034; OR, 0.04; 95% CI, 0.002 to 0.70; P=0.027) than in the control group.
Therapeutic hypothermia is the only proven treatment to reduce mortality and neurodevelopmental disabilities in patients with HIE. However, many infants with HIE have poor prognoses even after hypothermia treatment [2-4,16]. In addition, not all patients with HIE or birth asphyxia are eligible for therapeutic hypothermia because there is no available equipment or the patient does not meet the criteria of narrow indications (i.e., only for moderateto-severe HIE, ≥36 weeks of gestation, ≥1,800 to 2,000 g of birth weight, <6 hours of age, and without coagulopathy) [3,15]. In this retrospective study, we found that EPO administration significantly improved mortality and neurodevelopmental outcomes in neonates with HIE. We also showed that only 37.5% (21/56) of neonates with HIE received hypothermia treatment (Figure 1). Many infants with HIE could not receive hypothermia treatment because of the mild stage of HIE (37.5%, 21/56) and various causes, even if they had moderate-to-severe HIE (25%, 14/56). For those unable to receive hypothermia, no brain-focused treatment, rather only conventional intensive care, including respiratory support, fluid infusion, anticonvulsants, inotropic support, and correction of metabolic or electrolyte imbalance, was provided. In this regard, our results suggest that EPO could be considered an important therapeutic agent to provide neuroprotection with or without hypothermia.
EPO is a glycoprotein that is primarily responsible for red blood cell production [17]. Owing to this effect, it has been clinically used for decades to treat or prevent anemia of prematurity [18,19], and there have been no complications in neonates [20,21]. The key neuroprotective mechanism of EPO is known to involve the repair of the nervous system; EPO stimulates neurogenesis, oligodendrogenesis, and angiogenesis following hypoxic brain injury [22,23]. Additionally, EPO increases neuronal and glial migration around the injured area via the secretion of matrix metalloproteinases in in vitro studies [24,25]. Based on the results of animal studies that showed that the early administration of high-dose EPO was both neuroprotective and neurorestorative [22,26-28], EPO has emerged as an attractive treatment option for neonatal HIE since the early 2000s [13,29]. Since then, several studies on term or preterm infants with HIE have reported that EPO administration decreased mortality and improved long-term neurological outcomes [8,12,16,30-32]. Potential adverse effects of EPO include polycythemia and thrombosis. However, during more than 10 years of clinical trials in neonates, no obvious side effects or serious adverse events were observed with EPO administration [7,33]. Although EPO administration route, dosage, starting point, frequency of injections, and length of treatment vary, EPO appears to be a promising treatment modality for neonatal HIE without the risk of undesirable side effects. However, the prophylactic use of EPO for neuroprotection in preterm infants remains controversial. Although a meta-analysis of four studies performed in preterm infants showed cognitive improvement [32], a recent large-scale randomized trial regarding the use of prophylactic high-dose EPO in extremely preterm infants did not show advantages in terms of mortality or neurodevelopmental outcomes [34].
This study has some limitations. First, the data were collected from a single center and analyzed retrospectively. Second, the number of patients was small, especially for moderate-to-severe HIE. This might lower the power to prove the benefit of EPO on death or NDI in patients with moderate-to-severe HIE. Larger prospective studies should address this issue. Third, we included all patients with HIE, from mild to severe cases, rather than including only patients with moderate-to-severe HIE who received therapeutic hypothermia. As reported in this study, a significant number of neonatal HIE cases were not clinically indicated for therapeutic hypothermia. Because this study was not a randomized controlled study, we aimed to reflect real clinical situations. Fourth, EPO was administered off-label at the attending clinician’s discretion and not as per planned protocols. However, we decided to use EPO based on convincing evidence of the effectiveness of EPO for neonatal HIE, and EPO has been safely used for decades in infants. During the discussion with parents on EPO administration, every parent agreed to the off-label use of EPO, which could be beneficial in such a desperate state of their baby. Additionally, since off-label use of EPO is not costly, it is not a significant financial burden for most parents in Korea. We believe that now is not the time to discuss whether EPO should be used for neonatal HIE, but to determine the most effective dose, starting point, frequency, and length of EPO treatment for infants with HIE.
To our knowledge, this is the first study to investigate the clinical benefits of EPO administration for neonatal HIE in Korea. We demonstrated that EPO significantly reduced brain injuries in imaging studies and death or long-term NDI in neonatal HIE. We also found that a significant proportion of neonatal HIE cases were not clinically indicated for therapeutic hypothermia for various reasons. EPO can be valuable and, in some cases, the only treatment option to promote neuroprotection, especially for these infants. In conclusion, EPO is a promising therapeutic agent for neonatal HIE. Further large-scale prospective studies are required to address detailed guidelines for the optimal use of EPO for neonatal HIE.
ARTICLE INFORMATION
Ethical statement
The study was approved by the Institutional Review Board of Inha University Hospital (IRB no. 2022–05–017). The requirement for informed consent was waived by the board due to the retrospective nature of this study.
Table 1.
Comparisons of Neonates with Hypoxic-Ischemic Encephalopathy
Table 2.
Multivariable Logistic Regression Analysis
| Variable |
Total HIE* |
Moderate-to-severe HIE† |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Brain injury on imaging | 0.16 (0.03–0.92) | 0.040 | 0.04 (0.002–0.70) | 0.027 |
| Death or NDI | 0.09 (0.01–0.78) | 0.029 | 0.32 (0.03–3.70) | 0.360 |
REFERENCES
1. Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 2022;6:106–15.



2. Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed 2017;102:F346–58.



3. Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med 2012;166:558–66.

4. Sung IK. Therapeutic hypothermia for hypoxic-ischemic encephalopathy in newborn infants. Neonatal Med 2017;24:145–56.

5. Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res 2002;44:391–403.


6. Sun Y, Zhou C, Polk P, Nanda A, Zhang JH. Mechanisms of erythropoietin-induced brain protection in neonatal hypoxiaischemia rat model. J Cereb Blood Flow Metab 2004;24:259–70.


7. Oorschot DE, Sizemore RJ, Amer AR. Treatment of neonatal hypoxic-ischemic encephalopathy with erythropoietin alone, and erythropoietin combined with hypothermia: history, current status, and future research. Int J Mol Sci 2020;21:1487.



8. Razak A, Hussain A. Erythropoietin in perinatal hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinat Med 2019;47:478–89.


9. Juul SE, Comstock BA, Heagerty PJ, Mayock DE, Goodman AM, Hauge S, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial: background, aims, and study protocol. Neonatology 2018;113:331–8.



10. Mulkey SB, Ramakrishnaiah RH, McKinstry RC, Chang T, Mathur AM, Mayock DE, et al. Erythropoietin and brain magnetic resonance imaging findings in hypoxic-ischemic encephalopathy: volume of acute brain injury and 1-year neurodevelopmental outcome. J Pediatr 2017;186:196–9.


11. Park SH. Clinical trials for preterm infants’ neurodevelopment to the norm: erythropoietin and nutritional interventions. Neonatal Med 2017;24:101–9.

12. Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016;137:e20160191.


13. Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxicischemic encephalopathy. Pediatrics 2009;124:e218–26.


14. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 1976;33:696–705.


15. Jacobs SE. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. J Pediatr 2005;147:122–3.


16. Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed 2015;100:F541–52.



17. Linkenheimer WH, Grant WC, Berger H. Erythropoietin and known erythropoietic stimuli. Proc Soc Exp Biol Med 1960;104:230–2.


18. Halperin DS, Wacker P, Lacourt G, Felix M, Babel JF, Aapro M, et al. Effects of recombinant human erythropoietin in infants with the anemia of prematurity: a pilot study. J Pediatr 1990;116:779–86.


19. Ananthan A, Balasubramanian H, Rao S, Patole S. Clinical outcomes related to the gastrointestinal trophic effects of erythropoietin in preterm neonates: a systematic review and metaanalysis. Adv Nutr 2018;9:238–46.



20. Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012;10:CD004865.


21. Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2014;4:CD004863.

22. Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg 2010;113:598–608.



23. Gonzalez FF, Larpthaveesarp A, McQuillen P, Derugin N, Wendland M, Spadafora R, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke 2013;44:753–8.



24. Kaneko N, Kako E, Sawamoto K. Enhancement of ventricularsubventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives. Front Cell Neurosci 2013;7:235.



25. Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci 2006;26:5996–6003.



26. Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, et al. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab 2007;27:552–63.


27. Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci 2009;31:403–11.



28. Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. Beneficial effect of erythropoietin on sensorimotor function and white matter after hypoxia-ischemia in neonatal mice. Pediatr Res 2011;69:56–61.


29. Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics 2010;125:e1135–42.


30. Ohls RK, Cannon DC, Phillips J, Caprihan A, Patel S, Winter S, et al. Preschool assessment of preterm infants treated with darbepoetin and erythropoietin. Pediatrics 2016;137:e20153859.



31. Song J, Sun H, Xu F, Kang W, Gao L, Guo J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol 2016;80:24–34.



32. Fischer HS, Reibel NJ, Buhrer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a metaanalysis. Pediatrics 2017;139:e20164317.


-
METRICS

-
- 0 Crossref
- 2,694 View
- 100 Download
- Related articles in NM
-
Pharmacological Approaches in Newborn Infants with Hypoxic Ischemic Encephalopathy.2013 August;20(3)